Synthetic ‘bladder patch’ promotes tissue regeneration, restores function
A research team led by SQI member Arun Sharma has developed a synthetic, flexible “bladder patch” that outperformed the current standard surgery for severe bladder dysfunction in a long-term, large-animal model — the last step before beginning clinical trials.

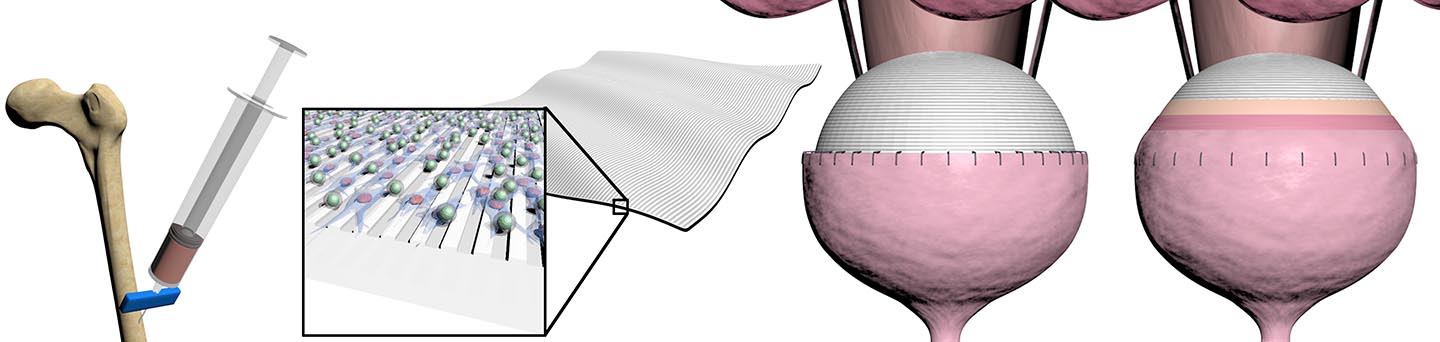
The “patch” is an elastic-like scaffold that is seeded with a patient’s own stem cells and then sutured to the bladder, where it helps to restore function and promote regeneration in the existing tissue. This novel platform has the potential to offer a better alternative to the current procedure, which involves removing a portion of the patient’s intestine and attaching it to the bladder.
This decades-old surgery enlarges the bladder and increases its capacity but is linked to a host of short- and long-term complications, including the interruption of the digestive tract, the need for repeat operations and other issues arising from the mismatch of bladder and intestinal tissue, such as infections with the potential of increasing cancer risk.
In a two-year study using a nonhuman primate model — the most clinically applicable to humans — the synthetic patch regenerated bladder tissue and restored the organ’s function. Compared to the intestinal patch method, the new approach was associated with significantly fewer complications during the follow-up period. The results were published online Jan. 30 as an advance article in PNAS Nexus.
“This highly translational, long-term study is paradigm-shifting for the field,” said Sharma, the corresponding author on the paper and Research Associate Professor of Urology and Biomedical Engineering at Northwestern. “It provides a unique and innovative means to regenerate bladder tissue for those that are suffering from severe bladder dysfunction, and we are excited to take the next step and pursue clinical trials for these patients.”
Millions of Americans have a dysfunctional bladder due to problems with their nerves, brain or spinal cord. These issues can arise from congenital defects such as spina bifida — where a person is born with a damaged spine — or traumatic injuries sustained at any point in life. When left untreated, severe bladder dysfunction causes routine infections and urination issues, and eventually leads to kidney damage, which in turn impacts the entire body.
Sharma envisions treating children with spina bifida in the initial clinical trial but hopes to eventually extend this solution to other patients with severe bladder dysfunction. He is the Director of Pediatric Urological Regenerative Medicine and Surgical Research at the Stanley Manne Children’s Research Institute at Lurie Children’s Hospital and has personally seen many patients he believes could benefit from the invention.
The scaffold is biodegradable, nontoxic and expands and contracts with the native bladder tissue due to its flexible nature. There are also no concerns with immune rejection, given that the cells it uses are extracted from a patient’s own bone marrow.
“This technology represents a significant advancement, as there are currently no other tissue engineering-based approaches available to these patients,” Sharma said. “I am confident this will help improve the quality of life of many patients who will now be able to avoid the use of intestinal tissues and its myriad complications.”
SQI members Guillermo Ameer and Earl Cheng, along with SQI scientific illustrator Mark Seniw, were coauthors of the study. The research was primarily supported by the National Institutes of Health (grants R01DK109539, R01EB026572 and T32-EB031527).
